Analysis of low-temperature CVD growth process of diamond films in C-H-F atmosphere
-
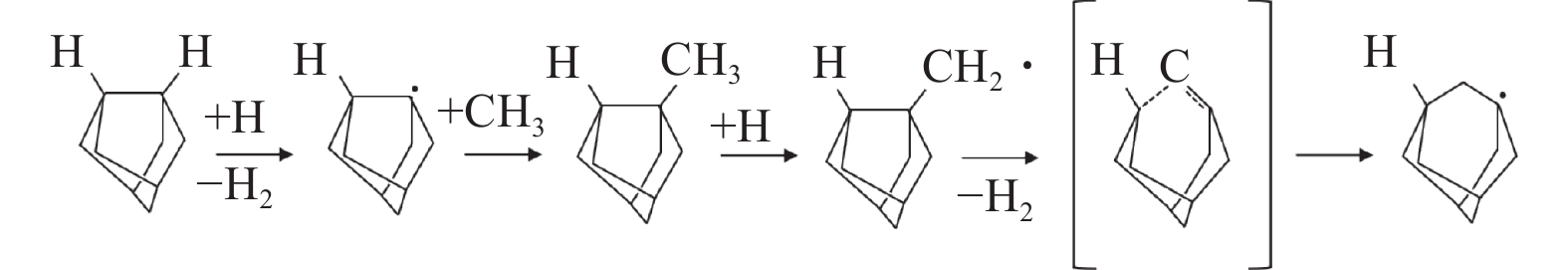
摘要: 基于第一性原理的密度泛函理论对C-H-F氛围下低温CVD金刚石薄膜的生长过程进行仿真分析,计算H、F原子在氢终止金刚石表面发生萃取反应的吸附能、反应热与反应能垒,并分析CF3、CF2、CF 3种生长基团在带有活性位点基底上的吸附。结果表明:与H原子相比,F原子更容易在氢终止金刚石表面萃出H,并以HF形式脱附,且在C-H-F氛围下有利于在低温时产生更多的活性位点;CF3、CF2、CF基团在吸附后的结构和吸附能绝对值都更有利于金刚石相的生成,适当提高CF3、CF2、CF基团的浓度有助于实现金刚石相的更高速率生长。Abstract: To better understand the growth mechanism of diamond films via low-temperature chemical vapor deposition in a C-H-F atmosphere, this paper employed density functional theory based on first principle. It calculated the adsorption energy, reaction heat, and reaction energy barrier of H and F atoms undergoing extraction reactions on the hydrogen-terminated diamond surface. Additionally, the analysis included the adsorption of CF3, CF2, and CF growth groups on the active site substrate. The results show that compared with H atoms, F atoms are more likely to extract H from the surface of hydrogen terminated diamond and desorb it in the form of HF. This process is advantageous for generating more active sites at low temperatures in a C-H-F atmosphere. Both the structure and the absolute value of the adsorption energy of CF3, CF2, and CF groups are more favorable for the generation of the diamond phase after adsorption. Increasing the concentration of CF3, CF2, and CF growth groups appropriately can facilitate the growth of diamond phase at a higher rate.
-
Key words:
- CVD diamond film /
- fluorine /
- deposition mechanism /
- first-principles /
- adsorption /
- surface chemical reaction
-
表 1 H、F原子在金刚石基底表面的吸附能
Table 1. Adsorption energy of H and F atoms on the diamond substrate surface
原子 Eads / eV H −0.170 8 F −2.092 8 表 2 CF3、CF2、CF基团吸附后的键长、键角值
Table 2. Bond length and bond angle values after adsorption of CF3, CF2, and CF groups
参数 取值 CF3 CF2 CF HC-CCFx吸附后键长 L2 / nm 0.162 2 0.161 9 0.161 0 HCC-CFx吸附后键长 L1 / nm 0.158 4 0.150 5 0.148 8 Fx-1C-F键长 L3 / nm 0.136 4 0.135 7 0.135 4 HCC键角 α / (°) 113.950 111.357 111.395 CCC键角 β / (°) 118.012 109.059 110.553 表 3 CF3、CF2、CF、CH3基团在生长基底的吸附能
Table 3. Adsorption energies of CF3, CF2, CF and CH3 groups on growth substrate
基团 吸附能 Eads / eV CF3 −3.7251 CF2 −3.4282 CF −3.5366 CH3 −3.3260 -
[1] LI X, XIA F, WANG C, et al. Deposition of an adherent diamond film on stainless steel using Cr/CrAlN as an interlayer [J]. Surface and Coatings Technology,2022,449:128960. doi: 10.1016/j.surfcoat.2022.128960 [2] SITTINGER V, BARON S, HOFER M, et al. Hot-filament CVD diamond coatings for optical applications [J]. Surface and Coatings Technology,2023,457:129287. doi: 10.1016/j.surfcoat.2023.129287 [3] HUA C, ZHANG X, CAI J, et al. The new application of boron-doped diamond film: Attenuator for high frequency and high power vacuum electronic devices [J]. Diamond and Related Materials,2022,124:108944. doi: 10.1016/j.diamond.2022.108944 [4] KROMKA A, POTOCKY Š, ČERMAK J, et al. Early stage of diamond growth at low temperature [J]. Diamond and Related Materials,2008,17(7/8/9/10):1252-1255. doi: 10.1016/j.diamond.2008.03.035 [5] PRABHAKARAN G S, DAS R, RAO M S R, et al. Temperature-dependent residual stress and thermal stability studies of multilayer HF-CVD diamond coatings on RB-SiC [J]. Surface and Coatings Technology,2022,441:128552. doi: 10.1016/j.surfcoat.2022.128552 [6] AN Q, CHENG M J, GODDARD III W A, et al. CCl radicals as a carbon source for diamond thin film deposition [J]. The Journal of Physical Chemistry Letters,2014,5(3):481-484. doi: 10.1021/jz402527y [7] TRAVA-AIROLDI V J, NOBREGA B N, CORAT E J, et al. Low temperature chemical vapour deposition of diamond on tungsten carbides using CF4 gas doping for machine tool applications [J]. Vacuum,1995,46(1):5-8. doi: 10.1016/0042-207X(95)80050-6 [8] SCHMIDT I, BENNDORF C. Investigations concerning the role of fluorine and chlorine in the low temperature growth of diamond [J]. Diamond and Related Materials,1997,6(8):964-969. doi: 10.1016/S0925-9635(96)00744-3 [9] GRANNEN K J, XIONG F, CHANG R P H. A comparison study of diamond films grown on tungsten carbide cobalt tool inserts with CH4 and CF4 gas sources [J]. Surface and Coatings Technology,1993,57(2/3):155-162. doi: 10.1016/0257-8972(93)90033-K [10] MUSALE D V, PAVASKAR N R, KSHIRSAGAR S T. Properties of diamond films grown from CH4, CF4 & H2 gas mixture using the hot-filament chemical vapor deposition technique [J]. Thin Solid Films,2002,422(1/2):20-27. doi: 10.1016/S0040-6090(02)00994-X [11] HENTSCHEL F, SCHMIDT I, BENNDORF C. Chemical vapour deposition of diamond at low substrate temperatures using fluorinated precursors [J]. Thin Solid Films,1996,290:196-199. [12] CORAT E J, TRAVA-AIROLDI V J, LEITE N F, et al. Diamond growth with CF4 addition in hot-filament chemical vapour deposition [J]. Journal of Materials Science,1997,32:941-947. doi: 10.1023/A:1018557817955 [13] RUDDER R A, POSTHILL J B, MARKUNAS R J. Thermal CVD of homoepitaxial diamond using CF4 and F2 [J]. Electronics Letters,1989,25(18):1220-1221. doi: 10.1049/el:19890818 [14] NYBERG T, HESZLER P, CARLSSON J O. Diamond deposition from halogenated methane precursors on Si and SiC substrates [J]. Diamond and Related Materials,1997,6(1):85-88. doi: 10.1016/S0925-9635(96)00586-9 [15] 简小刚, 王俊鹏, 何嘉诚. 不同反应气氛下氢终止金刚石表面的活化性能 [J]. 人工晶体学报,2019,48(3):73-79. doi: 10.3969/j.issn.1000-985X.2019.03.012JIAN Xiaogang, WANG Junpeng, He Jiacheng. Surface activation properties of the hydrogen-terminated diamond in different reaction atmospheres [J]. Journal of Synthetic Crystals,2019,48(3):73-79. doi: 10.3969/j.issn.1000-985X.2019.03.012 [16] PAULA D L B A, ORNELLAS F R. CASSCF and MRMP2 investigation of the interaction of arsenic adatoms with carbon dimers on the diamond (100)-2 × 1 surface [J]. Surface Science,2015,641:159-165. doi: 10.1016/j.susc.2015.06.018 [17] KANAI C, WATANABE K, TAKAKUWA Y. Ab initio calculations on etching of graphite and diamond surfaces by atomic hydrogen [J]. Physical Review B,2001,63(23):235311. doi: 10.1103/PhysRevB.63.235311 [18] SZNAJDER M. DFT-based modelling of carbon adsorption on the AlN surfaces and influence of point defects on the stability of diamond/AlN interfaces [J]. Diamond and Related Materials,2020,103:107694. doi: 10.1016/j.diamond.2020.107694 [19] MAEDA H, IRIE M, HINO T, et al. Diamond growth from a mixture of fluorocarbon and hydrogen in a microwave plasma [J]. Diamond and Related Materials,1994,3(7):1072-1078. doi: 10.1016/0925-9635(94)90120-1 [20] SCHMIDT I, HENTSCHEL F, BENNDORF C. Low temperature diamond growth using halogenated hydrocarbons [J]. Solid State Ionics,1997,101:97-101. [21] BISWAS B, SINGH P C. The role of fluorocarbon group in the hydrogen bond network, photophysical and solvation dynamics of fluorinated molecules [J]. Journal of Fluorine Chemistry,2020,235:109414. doi: 10.1016/j.jfluchem.2019.109414 [22] HUKKA T I, PAKKANEN T A, D'EVELYN M P. CF3 radicals as growth precursors and halogen-assisted growth on diamond (100) 2 × 1: An ab initio study [J]. Surface Science,1996,359(1/2/3):213-226. doi: 10.1016/0039-6028(96)00026-X [23] HUKKA T I, PAKKANEN T A, D'EVELVN M P. Chemisorption of fluorine, chlorine, HF and HCl on the diamond (100) 2x1 surface: An ab initio study [J]. The Journal of Physical Chemistry,1995,99(13):4710-4719. doi: 10.1021/j100013a048 -





 下载:
下载:











 邮件订阅
邮件订阅 RSS
RSS
