First-principles calculations of diamond/copper (silver, titanium carbide) interface properties
-
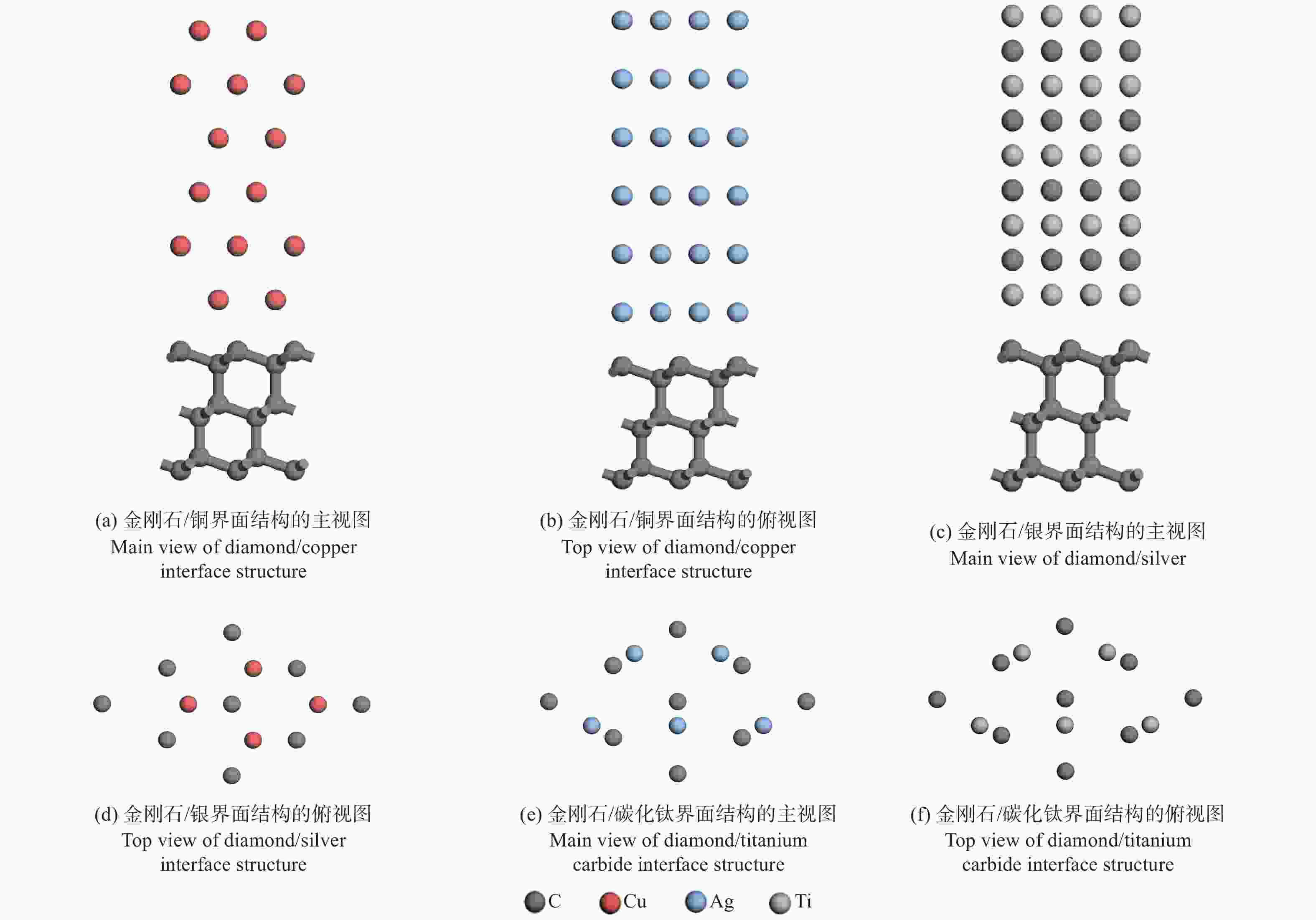
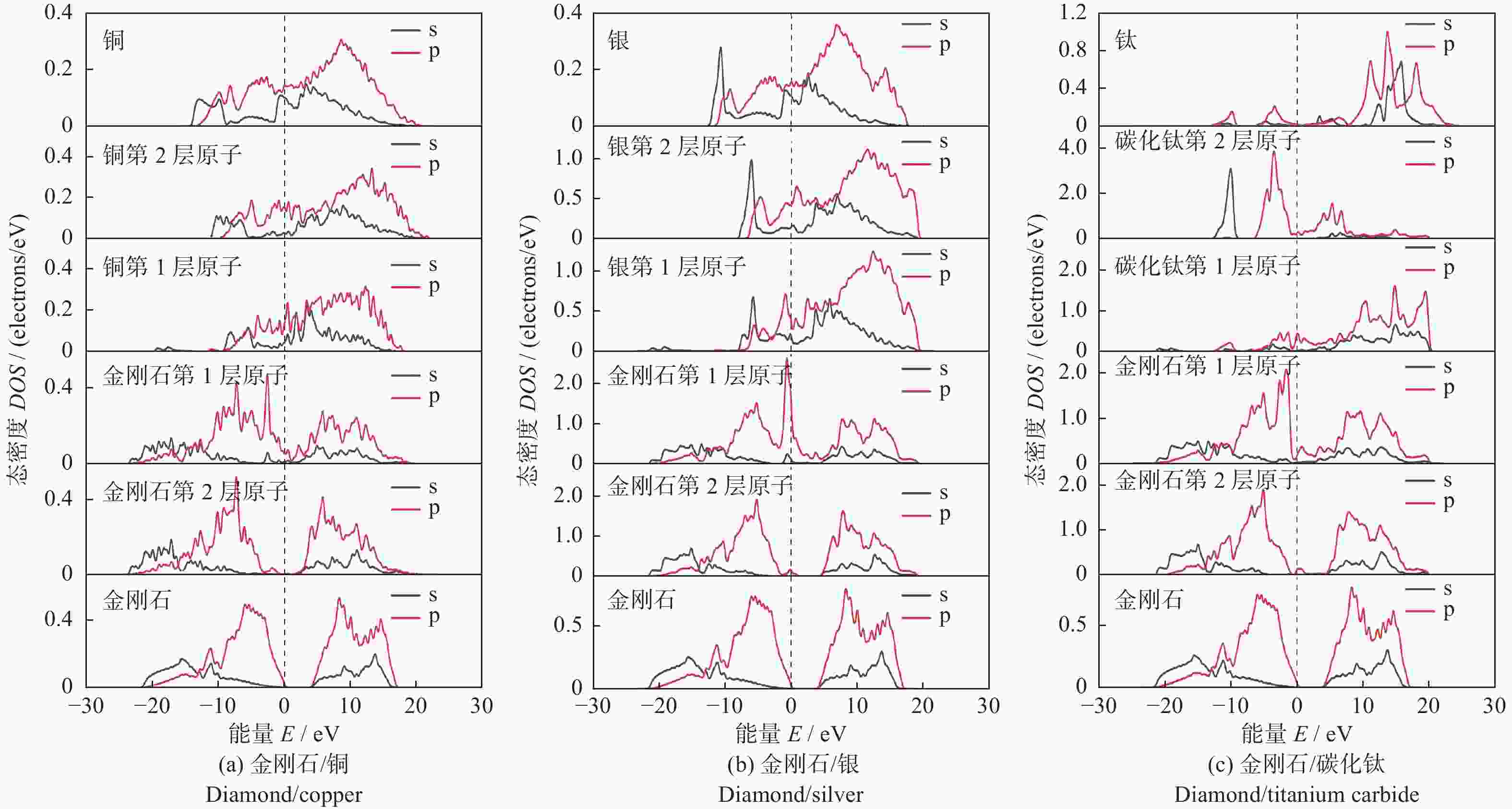
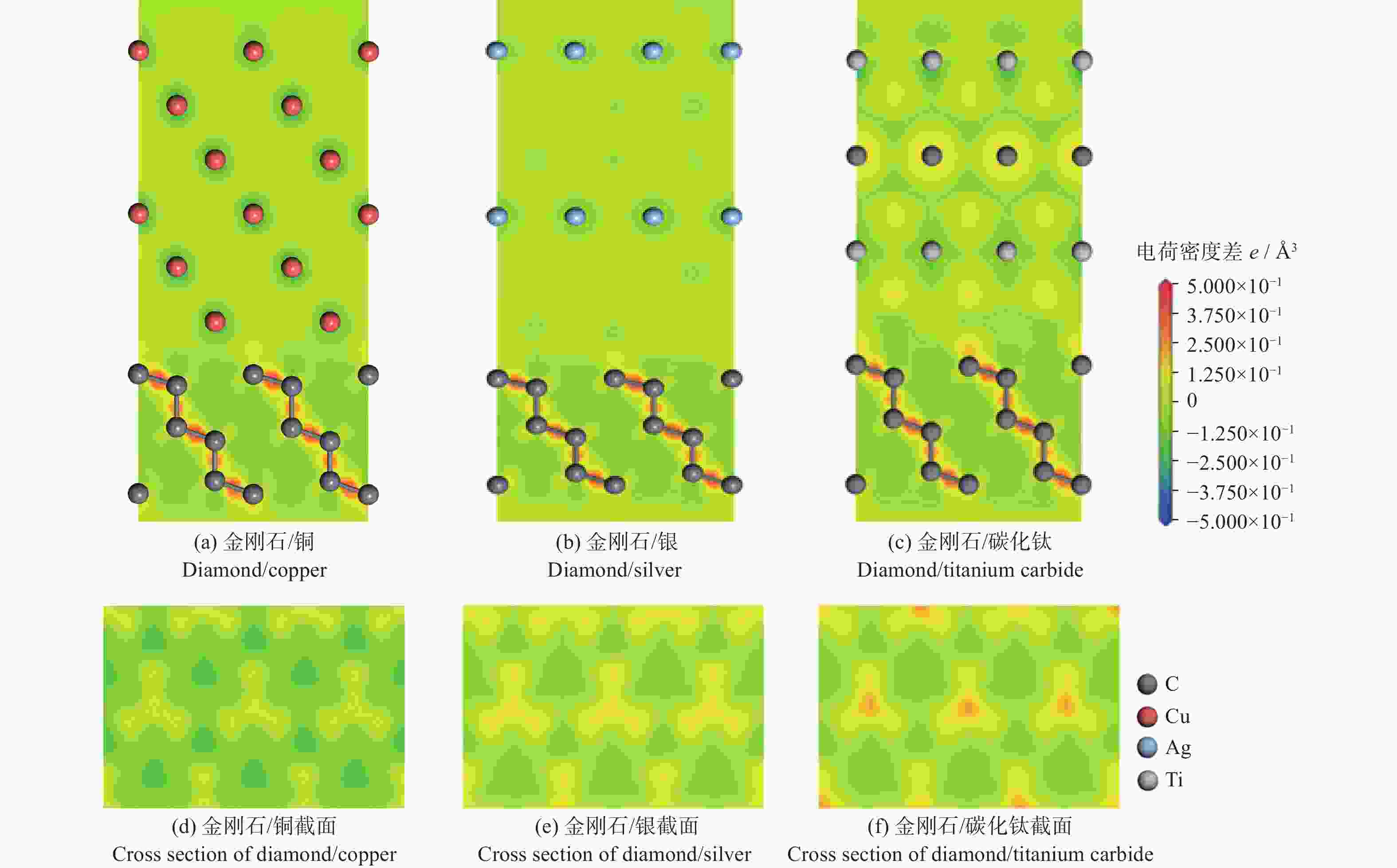
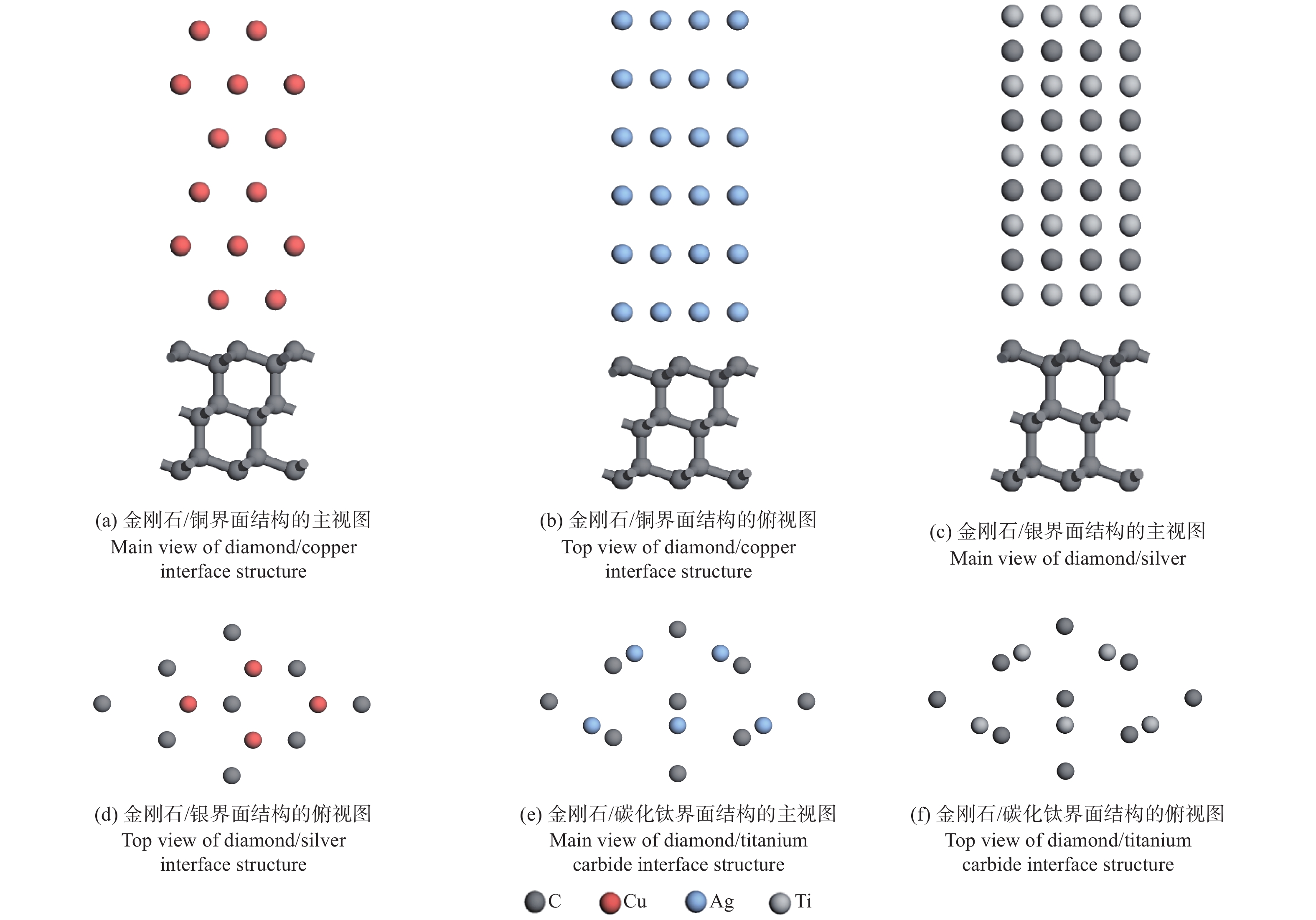
摘要: 采用第一性原理计算的方法研究金刚石/铜、金刚石/银、金刚石/碳化钛3种界面的结构、电子结构和传热。结果表明:金刚石/碳化钛的界面结构最为稳定,界面间距(1.990 Å)最小,界面黏附功(5.578 J/m2)最大,结合强度最高。电子态密度、马利肯布居分析、差分电荷密度、径向分布函数的结果表明金刚石/碳化钛存在较多的电荷转移和较强键合作用。 声子态密度的计算结果表明金刚石/碳化钛的界面热阻较低。
-
关键词:
- 金属基金刚石复合材料 /
- 第一性原理计算 /
- 界面结构 /
- 界面电子结构 /
- 界面传热
Abstract: The structure, electrical structure, and heat transmission of diamond/copper, diamond/silver, and diamond/titanium carbide surfaces have been investigated using first-principles calculations. The results show that the diamond/titanium carbide interfacial structure is the most stable, with the shortest interfacial distance (1.990 Å), the greatest interfacial adhesion effort (5.578 J/m2), and the best bond strength. The results of the electronic density of states, mulliken population analysis ,charge density difference, and radial distribution function indicate the presence of more charge transfer and stronger bonding in diamond/titanium carbide. According to the results of the phonon density calculation, the interfacial thermal resistance of diamond/titanium carbide is low. -
表 1 计算的铜、银、金刚石、碳化钛的晶胞参数以及他人试验和计算的结果
Table 1. Calculated cell parameters of copper, silver, diamond and titanium carbide, as well as the results of others’ experiments and calculations
表 2 碳化钛的表面原子弛豫
Table 2. Surface atomic relaxation of titanium carbide
间距
位置层间距变化 ξ / % 第3层
原子第5层
原子第7层
原子第9层
原子第11层
原子1/2 −10.49 22.61 −21.21 −21.21 −20.36 2/3 5.39 11.02 11.98 12.45 3/4 −4.93 −7.10 −7.06 4/5 0.49 1.87 5/6 −2.34 表 3 通过UBER和完全弛豫所得到的最佳界面间距和最佳黏附功
Table 3. Optimal interface spacing and adhesion work obtained by UBER and complete relaxation methods
结构名称UBER 完全弛豫 界面间距
d / Å界面黏附功
Wad / (J·m−2)界面间距
d / Å界面黏附功
Wad / (J·m−2)金刚石/铜 1.901 2.302 2.046 1.919 金刚石/银 2.128 3.920 2.236 4.291 金刚石/碳化钛 1.967 4.947 1.990 5.578 表 4 金刚石/铜界面原子轨道布居分析
Table 4. Analysis of atomic orbital population at diamond/copper interface
原子种类及位置 电荷量 Q / eV s p d 总数 差值 铜第2层铜原子 0.49 0.75 9.69 10.93 0.07 铜第1层铜原子 0.54 0.60 9.70 10.84 0.16 金刚石第1层碳原子 1.20 3.00 0.00 4.20 −0.20 金刚石第2层碳原子 1.14 2.92 0.00 4.06 −0.06 表 5 金刚石/银界面原子轨道布居分析
Table 5. Analysis of atomic orbital population at diamond/silver interface
原子种类及位置 电荷量 Q / eV s p d 总数 差值 银第2层银原子 0.59 0.58 9.78 10.95 0.05 银第1层银原子 0.60 0.43 9.75 10.78 0.22 金刚石第1层碳原子 1.18 2.98 0.00 4.16 −0.16 金刚石第2层碳原子 1.13 2.93 0.00 4.06 −0.06 表 6 金刚石/碳化钛界面原子轨道布居分析
Table 6. Atomic orbital population analysis of diamond/titanium carbide interface
原子种类及位置 电荷量 Q / eV s p d 总数 差值 碳化钛第2层碳原子 1.47 3.25 0.00 4.72 −0.72 碳化钛第1层钛原子 2.18 6.36 2.64 11.19 0.81 金刚石第1层碳原子 1.20 3.08 0.00 4.27 −0.27 金刚石第2层碳原子 1.15 2.94 0.00 4.08 −0.08 -
[1] MOORE A L, SHI L. Emerging challenges and materials for thermal management of electronics [J]. Materials Today,2014,17(4):163-174. doi: 10.1016/j.mattod.2014.04.003 [2] AZINA C, CORNU I, SILVAIN J F, et al. Effect of titanium and zirconium carbide interphases on the thermal conductivity and interfacial heat transfers in copper/diamond composite materials [J]. AIP Advances,2019,9(5):055315. doi: 10.1063/1.5052307 [3] BAI G, ZHANG Y, DAI J, et al. Tunable coefficient of thermal expansion of Cu-B/diamond composites prepared by gas pressure infiltration [J]. Journal of Alloys and Compounds,2019,794:473-481. doi: 10.1016/j.jallcom.2019.04.252 [4] JIA J, BAI S, XIONG D, et al. Effect of tungsten based coating characteristics on microstructure and thermal conductivity of diamond/Cu composites prepared by pressureless infiltration [J]. Ceramics International,2019,45(8):10810-10818. doi: 10.1016/j.ceramint.2019.02.156 [5] MOLINA-JORDÁ J M. Thermal conductivity of metal matrix composites with coated inclusions: A new modelling approach for interface engineering design in thermal management [J]. Journal of Alloys and Compounds,2018,745:849-855. doi: 10.1016/j.jallcom.2018.02.092 [6] CHE Z, LI J, WANG Q, et al. The formation of atomic-level interfacial layer and its effect on thermal conductivity of W-coated diamond particles reinforced Al matrix composites [J]. Composites Part A: Applied Science and Manufacturing,2018,107:164-170. doi: 10.1016/j.compositesa.2018.01.002 [7] CIUPIŃSKI Ł, KRUSZEWSKI M J, GRZONKA J, et al. Design of interfacial Cr3C2 carbide layer via optimization of sintering parameters used to fabricate copper/diamond composites for thermal management applications [J]. Materials & Design,2017,120:170-185. [8] CHEN H, JIA C, LI S. Interfacial characterization and thermal conductivity of diamond/Cu composites prepared by two HPHT techniques [J]. Journal of Materials Science,2012,47(7):3367-3375. doi: 10.1007/s10853-011-6180-6 [9] ARAI S, UEDA M. Fabrication of high thermal conductivity copper/diamond composites by electrodeposition under potentiostatic conditions [J]. Journal of Applied Electrochemistry,2020,50(5):631-638. doi: 10.1007/s10800-020-01414-3 [10] LI H, WANG C, WU L, et al. Optimization of process parameters, microstructure, and thermal conductivity properties of Ti-coated diamond/copper composites prepared by spark plasma sintering [J]. Journal of Materials Science: Materials in Electronics,2021,32(7):9115-9125. doi: 10.1007/s10854-021-05579-1 [11] WU X, WAN D, ZHANG W, et al. Constructing efficient heat transfer channels at the interface of diamond/Cu composites [J]. Composite Interfaces,2021,28(6):625-635. doi: 10.1080/09276440.2020.1795466 [12] JHONG Y S, TSENG H T, LIN S J. Diamond/Ag-Ti composites with high thermal conductivity and excellent thermal cycling performance fabricated by pressureless sintering [J]. Journal of Alloys and Compounds,2019,801:589-595. doi: 10.1016/j.jallcom.2019.06.167 [13] TANG Y, WANG L, ZHAO C. Enhancement of the thermal properties of silver-diamond composites with chromium carbide coating [J]. Applied Physics A,2014,115(2):379-385. doi: 10.1007/s00339-014-8254-1 [14] CHEN L, CHEN S, HOU Y. Understanding the thermal conductivity of diamond/copper composites by first-principles calculations [J]. Carbon,2019,148:249-257. doi: 10.1016/j.carbon.2019.03.051 [15] XIE H, CHEN Y, ZHANG T, et al. Adhesion, bonding and mechanical properties of Mo doped diamond/Al (Cu) interfaces: A first principles study [J]. Applied Surface Science,2020,527:146817. doi: 10.1016/j.apsusc.2020.146817 [16] SEGALL M D, LINDAN P J D, PROBERT M J, et al. First-principles simulation: Ideas, illustrations and the CASTEP code [J]. Journal of Physics: Condensed Matter,2002,14(11):2717-2744. doi: 10.1088/0953-8984/14/11/301 [17] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism [J]. Physical Review B,1990,41(11):7892. doi: 10.1103/PhysRevB.41.7892 [18] WU Z, COHEN R E. More accurate generalized gradient approximation for solids [J]. Physical Review B,2006,73(23):235116. doi: 10.1103/PhysRevB.73.235116 [19] CHADI D J. Special points for Brillouin-zone integrations [J]. Physical Review B,1977,16(4):1746. doi: 10.1103/PhysRevB.16.1746 [20] HEAD J D, ZERNER M C. A broyden-fletcher-goldfarb-shanno optimization procedure for molecular geometries [J]. Chemical Physics Letters,1985,122(3):264-270. doi: 10.1016/0009-2614(85)80574-1 [21] BARZILAI J, BORWEIN J M. Two-point step size gradient methods [J]. IMA Journal of Numerical Analysis,1988,8(1):141-148. doi: 10.1093/imanum/8.1.141 [22] KIM W J, XING S, KREMER G, et al. Electronic structure of heavy halogen atoms adsorbed on the Cu (111) surface: A combined ARPES and first principles calculations study [J]. The Journal of Physical Chemistry C,2019,123(43):26309-26314. doi: 10.1021/acs.jpcc.9b07057 [23] VILLARS P, DAAMS J L C. Atomic-environment classification of the chemical elements [J]. Journal of Alloys and Compounds,1993,197(2):177-196. doi: 10.1016/0925-8388(93)90041-K [24] QIAN H Q, MAO H Y, SONG F, et al. Study on the adsorption of fluorescein on Ag (110) substrate [J]. Applied Surface Science,2010,256(9):2686-2690. doi: 10.1016/j.apsusc.2009.10.056 [25] LIU L, Bassett W A. Compression of Ag and phase transformation of NaCl [J]. Journal of Applied Physics,1973,44(4):1475-1479. doi: 10.1063/1.1662396 [26] GU K, PANG M, ZHAN Y. Insight into interfacial structure and bonding nature of diamond (001)/Cr3C2 (001) interface [J]. Journal of Alloys and Compounds,2019,770:82-89. doi: 10.1016/j.jallcom.2018.08.112 [27] OWNBY P D, YANG X, LIU J. Calculated X‐ray diffraction data for diamond polytypes [J]. Journal of the American Ceramic Society,1992,75(7):1876-1883. doi: 10.1111/j.1151-2916.1992.tb07211.x [28] KHANZADEH M, ALAHYARIZADEH G. A DFT study on pressure dependency of TiC and ZrC properties: Interconnecting elastic constants, thermodynamic, and mechanical properties [J]. Ceramics International,2021,47(7):9990-10005. doi: 10.1016/j.ceramint.2020.12.145 [29] RAZUMOVSKIY V I, POPOV M N, DING H, et al. Formation and interaction of point defects in group IVb transition metal carbides and nitrides [J]. Computational Materials Science,2015,104:147-154. doi: 10.1016/j.commatsci.2015.03.042 [30] SUN Q, WEI Y, ZHANG J, et al. Effect of lattice mismatch on HgCdTe LPE film surface morphology [J]. Journal of Electronic Materials,2016,45(9):4674-4679. doi: 10.1007/s11664-016-4637-8 -



 下载:
下载:




 邮件订阅
邮件订阅 RSS
RSS
