Research progress and prospect of high entropy oxide
-
摘要: 高熵氧化物是由5种或5种以上等摩尔比的氧化物合成出的单相结构稳定固溶体,其具有优异的热学、磁学、电学及抗腐蚀性能等。目前的研究主要集中在对高熵氧化物现有性能的深度发掘和拓展,以及基于其优异性能在锂离子电池电极材料、介电材料、磁性材料和催化材料等方面的应用上。综述高熵氧化物的分类、制备方法以及性能特点,并对高熵氧化物的发展方向进行分析和展望。Abstract: High entropy oxide ceramics are stable solid solutions with single-phase structure, which are synthesized from five or more oxides with equal molar ratio and have excellent thermal, magnetic, electrical and corrosion resistance properties. At present, the researches mainly focus on the in-depth exploration and expansion of the existing properties of high entropy oxide ceramics, as well as their applications based on its excellent properties in lithium ion battery electrode materials, dielectric materials, magnetic materials and catalytic materials, etc. In this paper, the classification, the preparation methods and the properties of high entropy oxide ceramics are reviewed, and the development direction of high entropy oxide ceramics is analyzed and prospected.
-
Key words:
- high entropy oxides /
- preparation method /
- performance and application
-
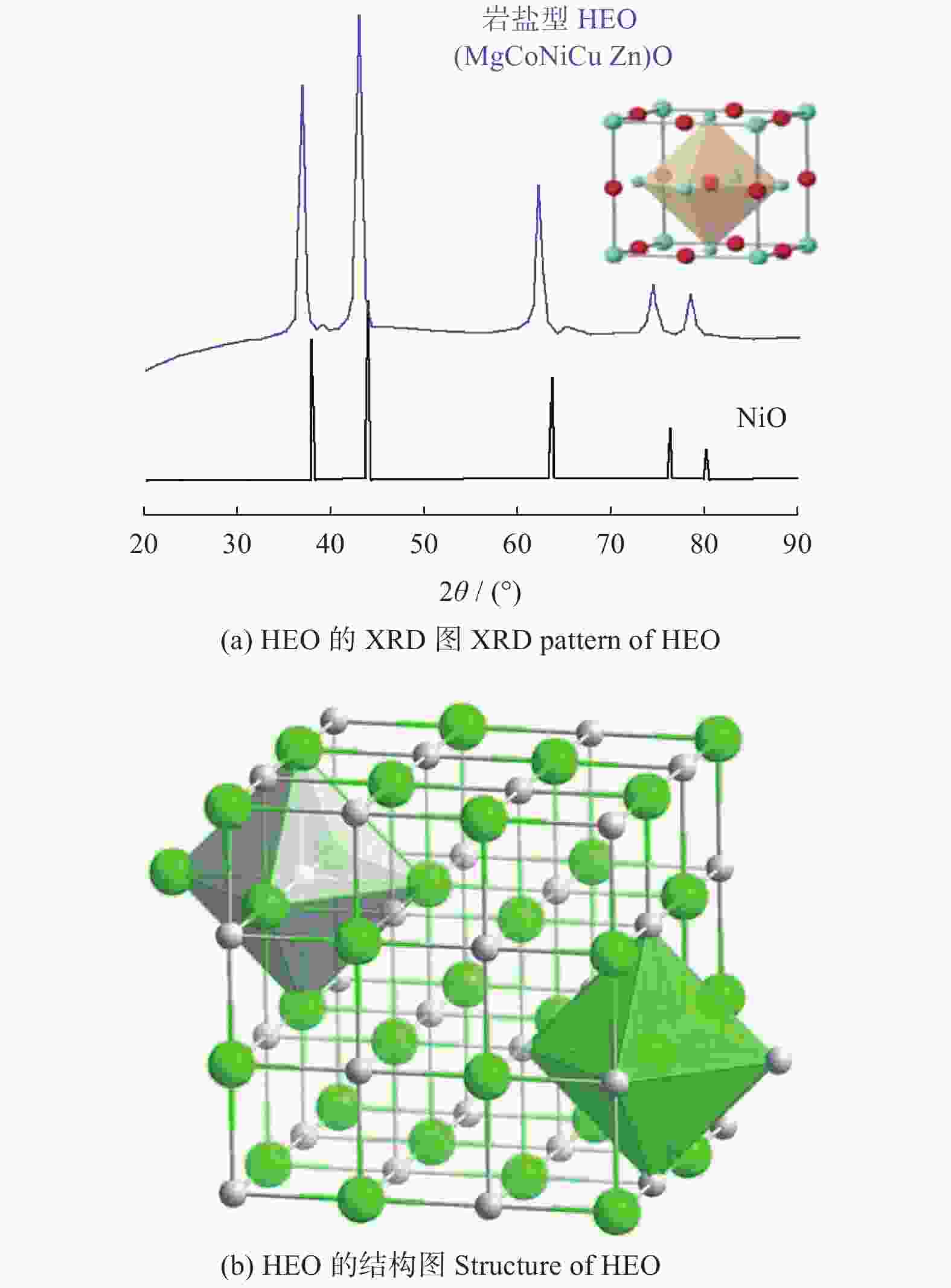
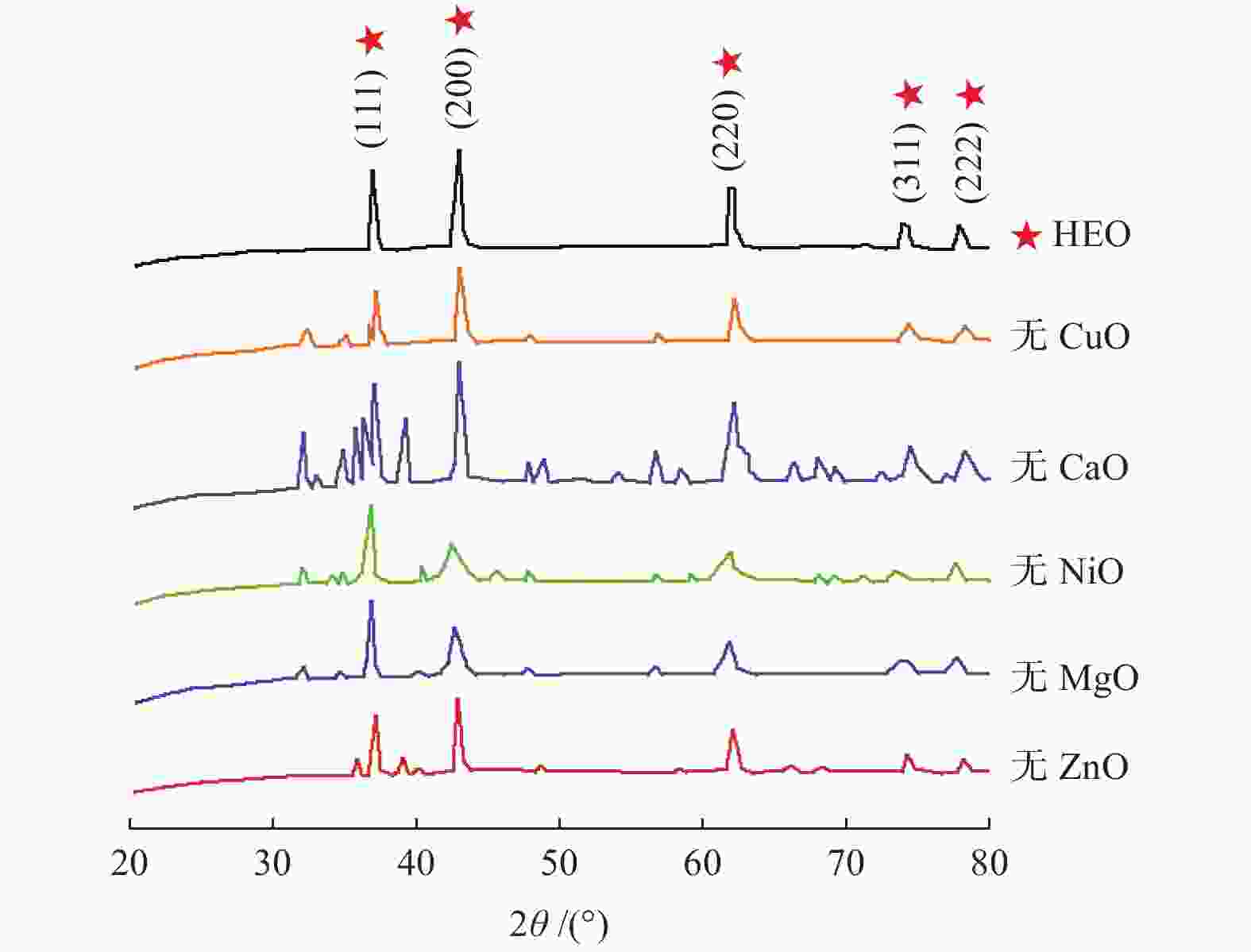
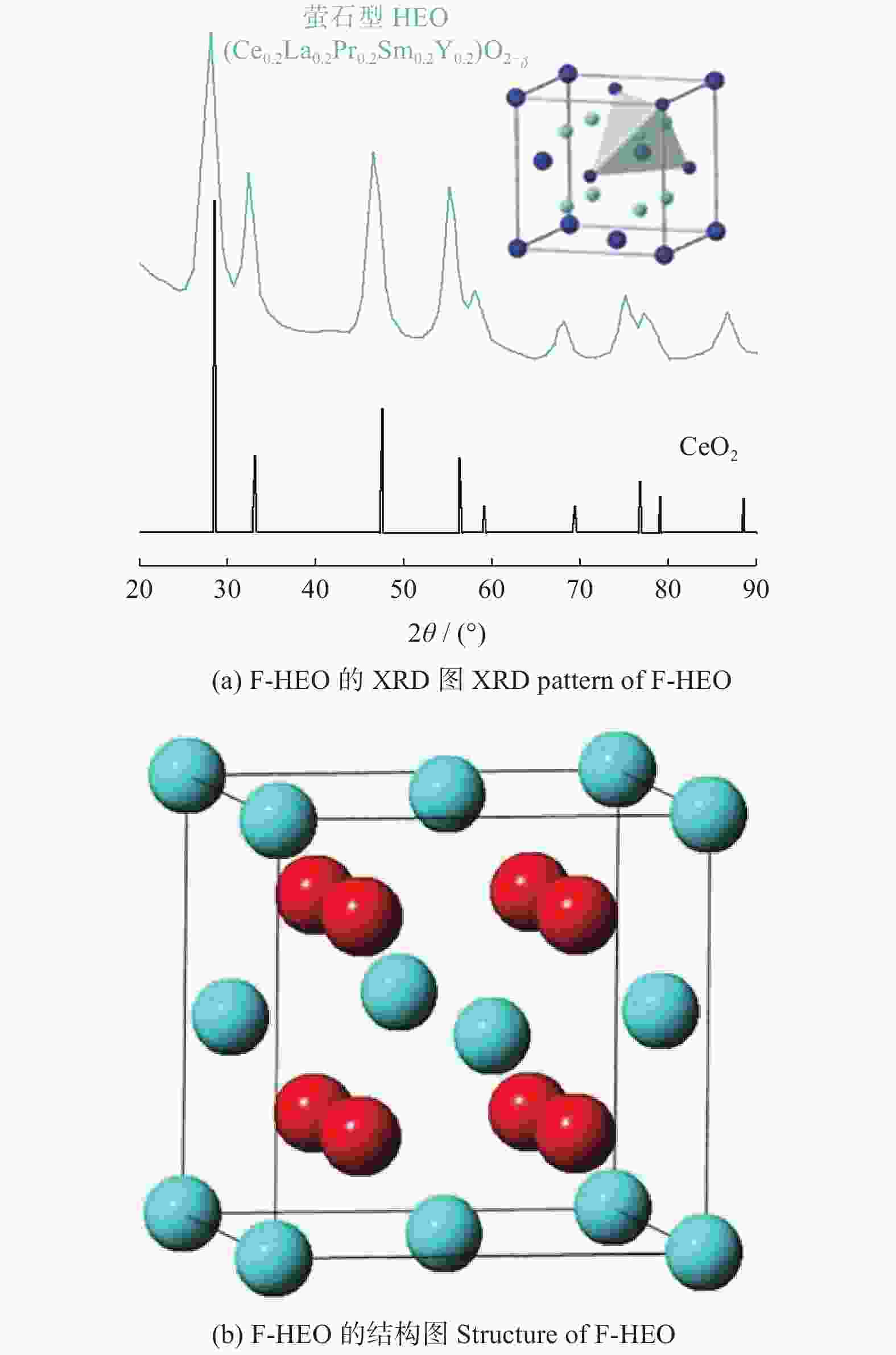
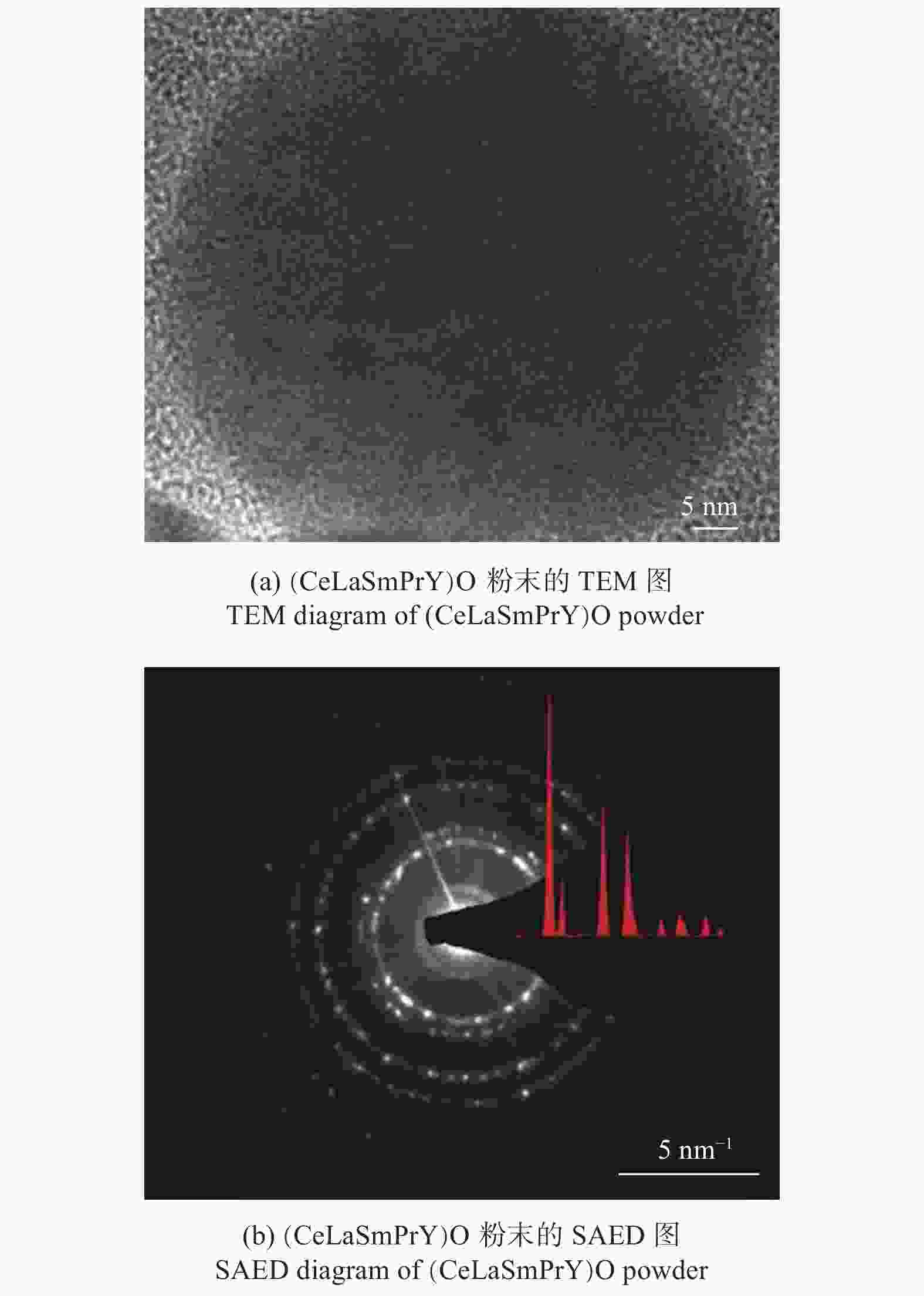
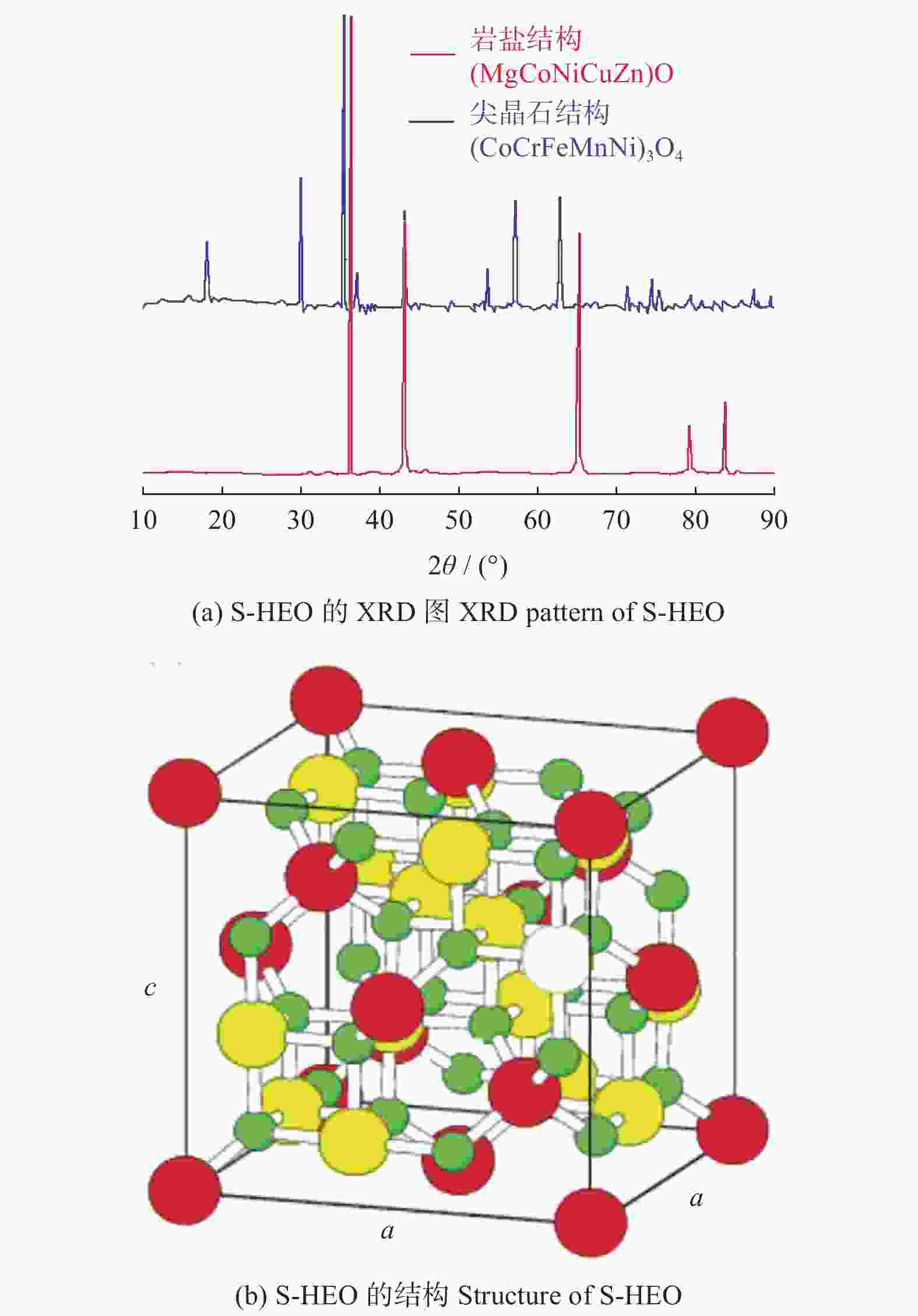
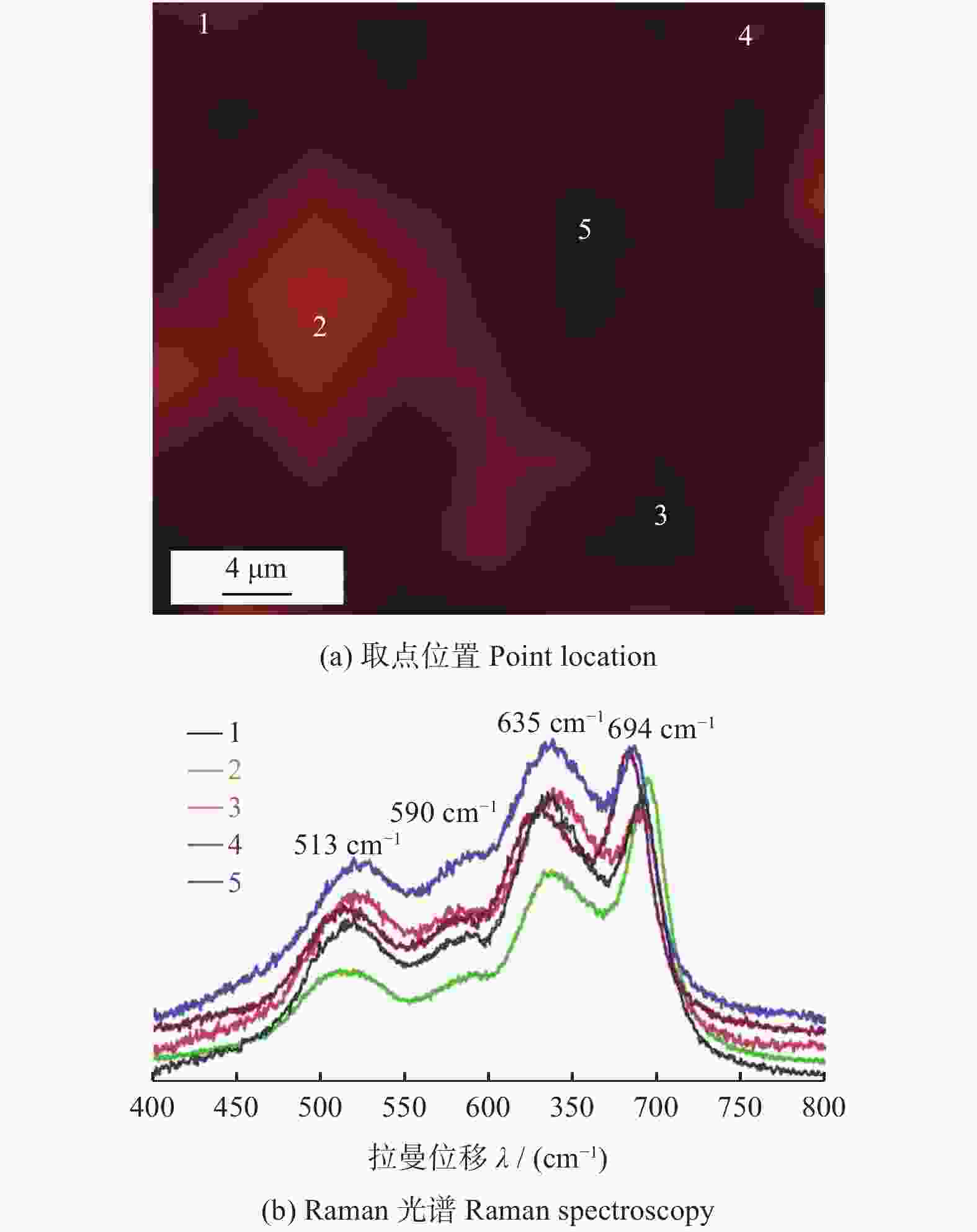
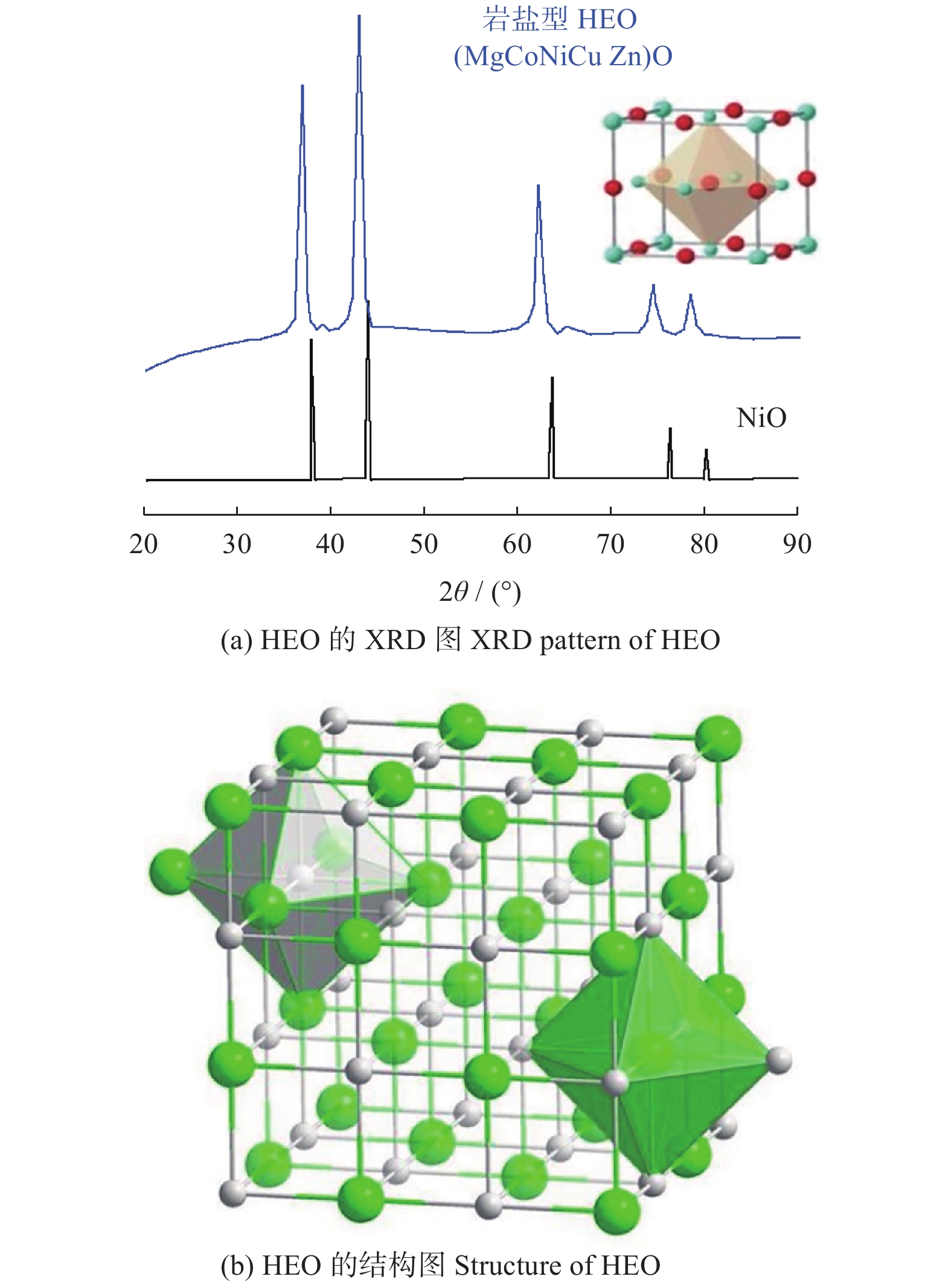
图 7 S - HEO的XRD[25]和结构图
Figure 7. XRD pattern and structure of S - HEO
表 1 高熵氧化物的发展趋势
Table 1. Development trend of high entropy oxides
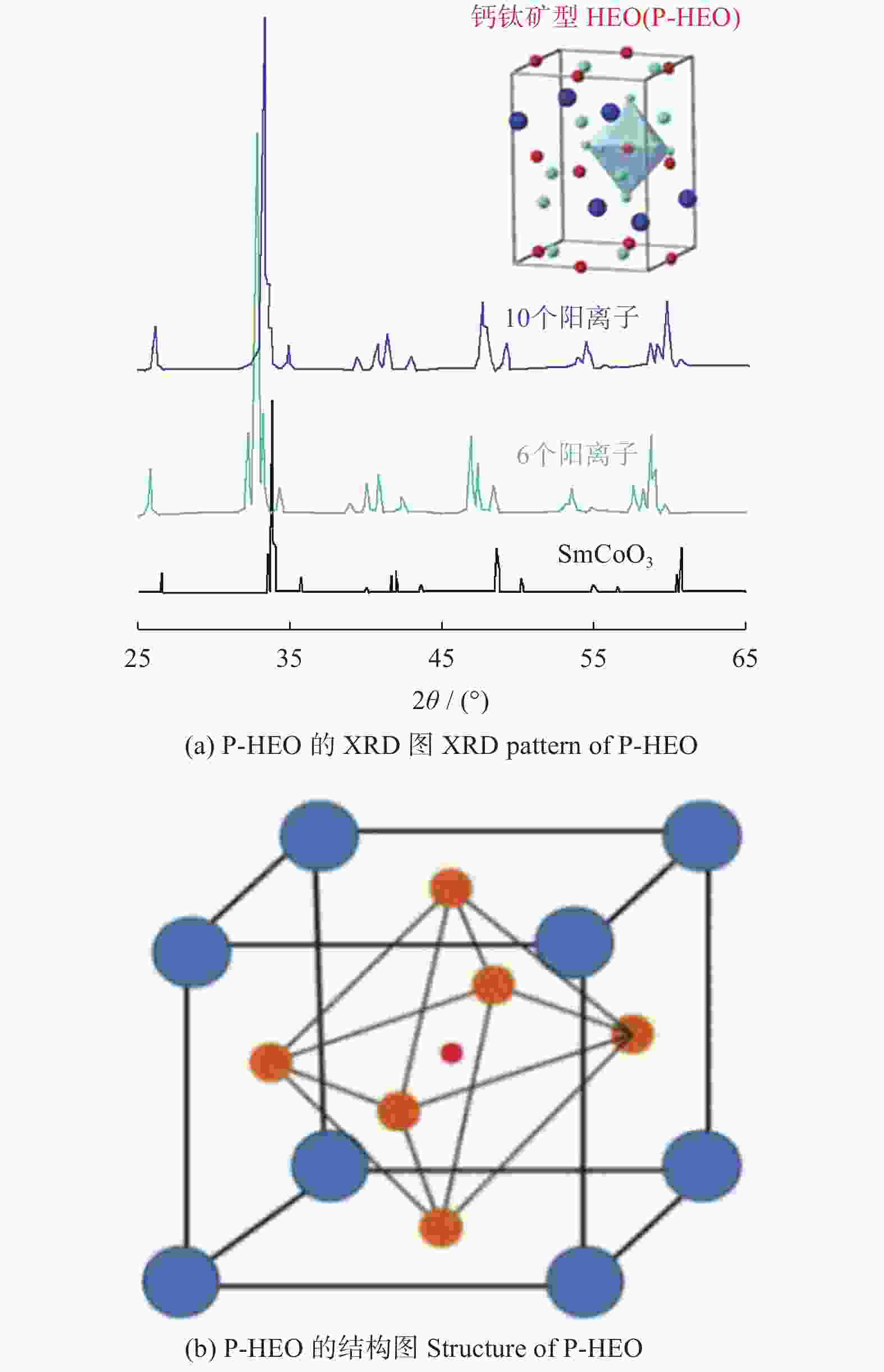
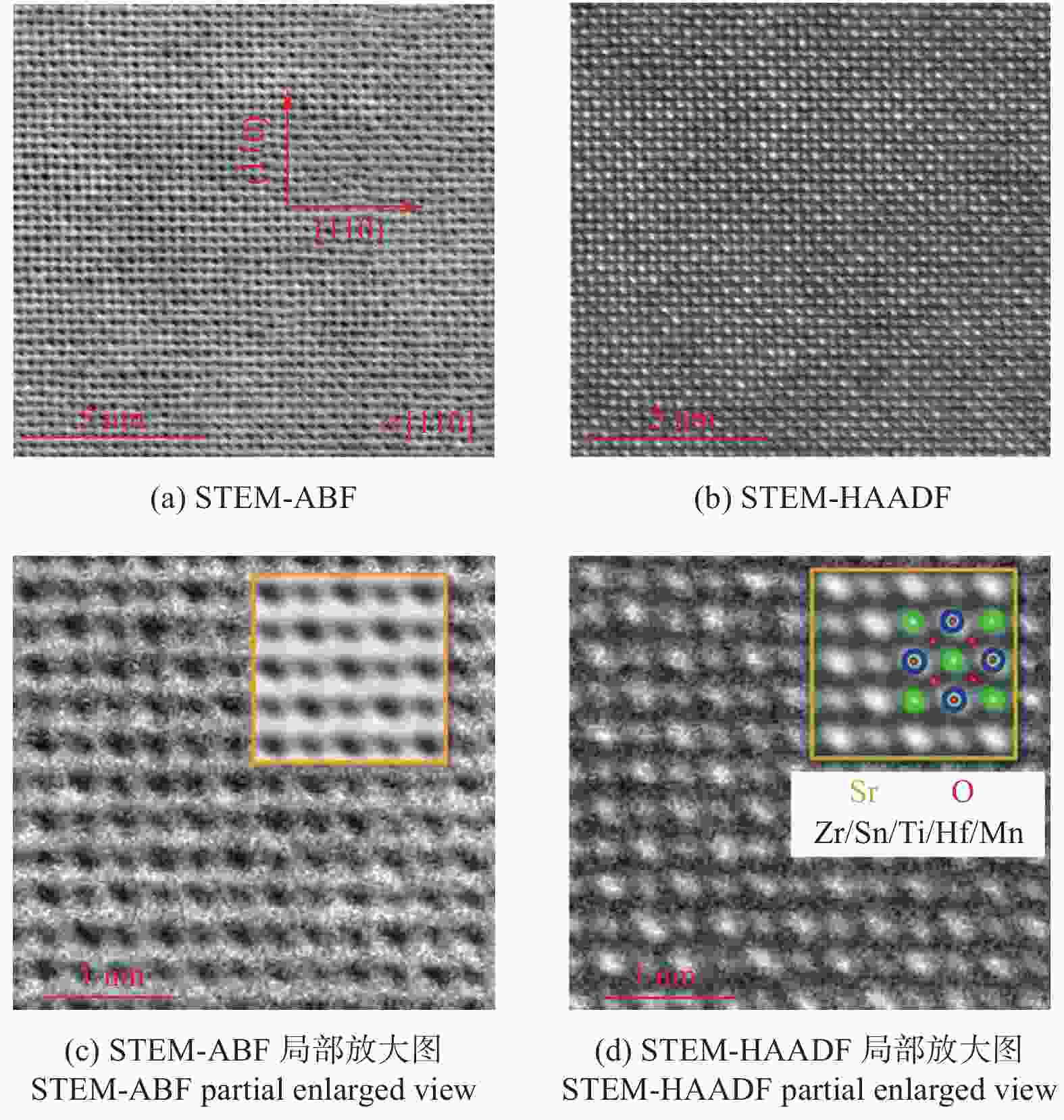
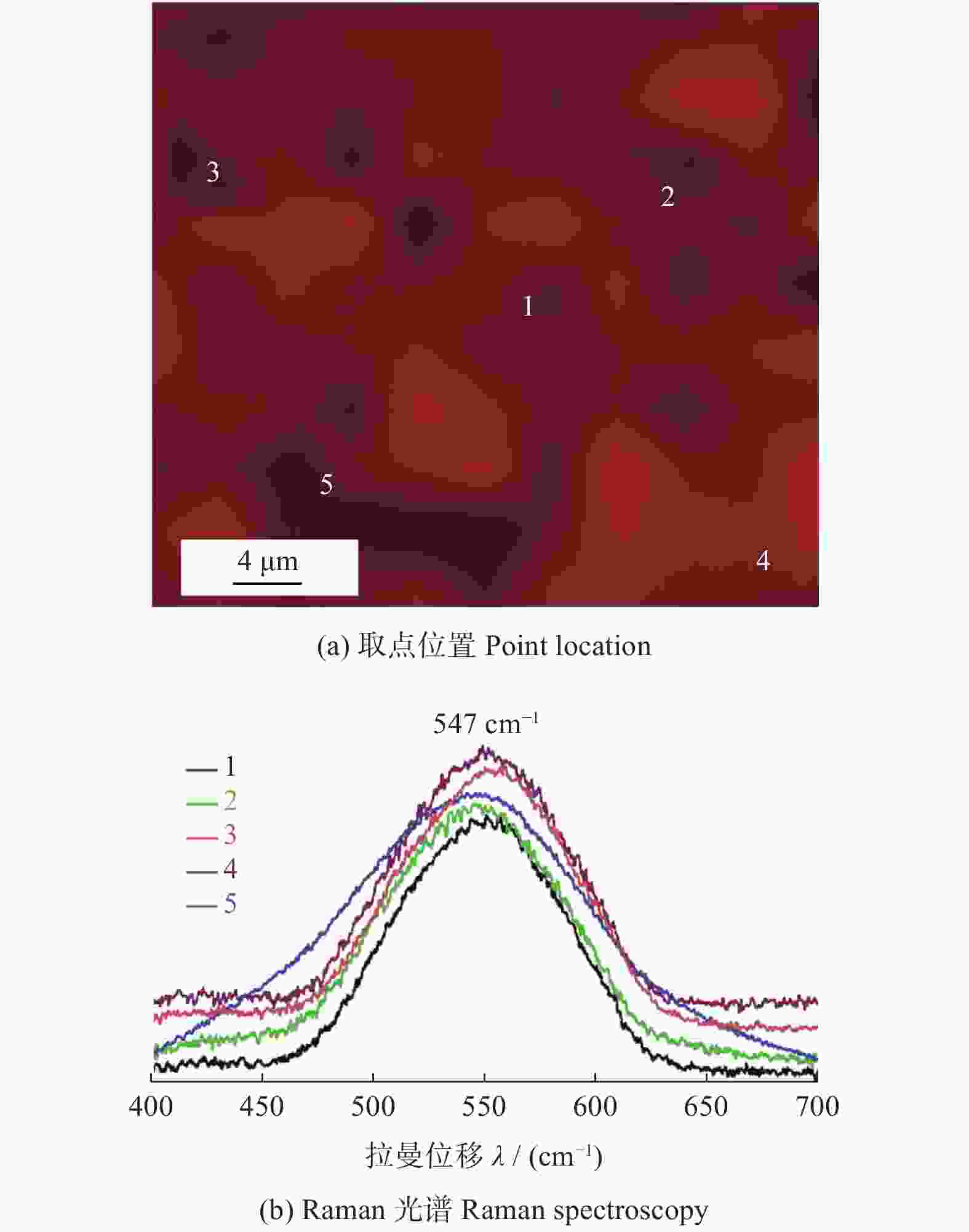
年份 相应进展 2015 首次提出熵稳定的氧化物,利用固相反应法制备岩盐型(MgCoNiCuZn)O 2016 (MgCoNiCuZn)O电学性能探究,加入Li+离子提升其电学性能 2017 (MgCoNiCuZn)O微观结构及内部畸变机理研究,喷雾热解法制备萤石型(CeLaPrSmY)O+(Re) 2018 喷雾热解法、湿法合成(MgCoNiCuZn)O,可用于能量储存和催化CO。稀土钙钛矿型、
萤石型(Ce0.2Zr0.2Hf0.2Sn0.2Ti0.2)O2和尖晶石型(CoCrFeMnNi)3O4高熵氧化物的制备2019 研究(MgCoNiCuZn)O的热学、磁和力学性能 2020~至今 (MgCoNiZn)1-xLixO电化学性能研究,(CoCrFeMnNi)3O4的微观结构及性能探究 -
[1] YEH J, CHEN S, LIN S, et al. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes [J]. Advanced Engineering Materials,2004,6(5):299-303. doi: 10.1002/adem.200300567 [2] ROST C, SACHET E, BORMAN T, et al. Entropy-stabilized oxides [J]. Nature Communications,2015,6(1):1-8. [3] GILD J, ZHANG Y, HARRINGTON T, et al. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultrahigh temperature ceramics [J]. Scientific Reports,2016,6(1):1-10. doi: 10.1038/s41598-016-0001-8 [4] SARKER P, HARRINGTON T, TOHER C, et al. High-entropy high-hardness metal carbides discovered by entropy descriptors [J]. Nature Communications,2018,9:4980. doi: 10.1038/s41467-018-07160-7 [5] JOHANSSON K, RIEKDHR L, FRITZE S, et al. Multicomponent Hf-Nb-Ti-V-Zr nitride coatings by reactive magnetron sputter deposition [J]. Surface and Coatings Technology,2018,349:529-539. doi: 10.1016/j.surfcoat.2018.06.030 [6] QIN Y, LIU J, LI F, et al. A high entropy silicide by reactive spark plasma sintering [J]. Journal of Advanced Ceramics,2019,8(1):148-152. doi: 10.1007/s40145-019-0319-3 [7] ZHANG R, GUCCI F, ZHU H, et al. Data-driven design of ecofriendly thermoelectric high-entropy sulfides [J]. Inorganic Chemistry,2018,57(20):13027-13033. doi: 10.1021/acs.inorgchem.8b02379 [8] BERARDAN D, FRANGER S, DRAGEO D, et al. Colossal dielectric constant in high entropy oxides [J]. Rapid Research Letters,2016,10(4):328-333. [9] OSES C, TOHER C, CURTAROLO S. High-entropy ceramics [J]. Nature Reviews Materials,2020,5(4):295-309. doi: 10.1038/s41578-019-0170-8 [10] SARKAR A, WANG Q, SCHIELE A, et al. High-entropy oxides: Fundamental aspects and electrochemical properties [J]. Advanced Materials,2019,31(26):201806236. [11] BERARDAN D, FRANGER S, MEENA A, et al. Room temperature lithium superionic conductivity in high entropy oxides [J]. Journal of Materials Chemistry A,2016,4(24):9536-9541. doi: 10.1039/C6TA03249D [12] WANG Q, SARKAR A, WANG D, et al. Multi-anionic and-cationic compounds: New high entropy materials for advanced Li-ion batteries [J]. Energy & Environmental Science,2019,12(8):2433-2442. [13] WANG Q, SARKAR A, LI Z, et al. High entropy oxides as anode material for Li-ion battery applications: A practical approach [J]. Electrochemistry Communications,2019,100:121-125. doi: 10.1016/j.elecom.2019.02.001 [14] QIU N, CHEN H, YANG Z, et al. A high entropy oxide (Mg0. 2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance [J]. Journal of Alloys and Compounds,2019,777:767-774. doi: 10.1016/j.jallcom.2018.11.049 [15] SEGURA M, TAKAYAMA T, BERARDAN D, et al. Long-range magnetic ordering in rocksalt-type high-entropy oxides [J]. Applied Physics Letters,2019,114(12):122401. doi: 10.1063/1.5091787 [16] ZHANG J, YAN J, CALDER S, et al. Long-range antiferromagnetic order in a rocksalt high entropy oxide [J]. Chemistry of Materials,2019,31(10):3705-3711. doi: 10.1021/acs.chemmater.9b00624 [17] PARK M, HWANG C. Fluorite-structure antiferroelectrics [J]. Reports on Progress in Physics,2019,82(12):124502. doi: 10.1088/1361-6633/ab49d6 [18] SARKAR A, LOHO C, VELASCO L, et al. Multicomponent equiatomic rare earth oxides with a narrow band gap and associated praseodymium multivalency [J]. Dalton Transactions,2017,46(36):12167-12176. doi: 10.1039/C7DT02077E [19] DJENADIC R, SARKAR A, LEMENS O, et al. Multicomponent equiatomic rare earth oxides [J]. Materials Research Letters,2017,5(2):102-109. doi: 10.1080/21663831.2016.1220433 [20] 王晓鹏, 孔凡涛. 高熵合金及其他高熵材料研究新进展 [J]. 航空材料学报,2019,39(6):1-19.WANG Xiaopeng, KONG Fantao. Resent development in high-entropy alloys and other high-entropy materials [J]. Journal of Aeronautical Materials,2019,39(6):1-19. [21] CHEN K, PEI X, TANG L, et al. A five-component entropy-stabilized fluorite oxide [J]. Journal of the European Ceramic Society,2018,38(11):4161-4164. doi: 10.1016/j.jeurceramsoc.2018.04.063 [22] SARKAR A, DJENADIC R, WANG D, et al. Rare earth and transition metal based entropy stabilised perovskite type oxides [J]. Journal of the European Ceramic Society,2018,38(5):2318-2327. doi: 10.1016/j.jeurceramsoc.2017.12.058 [23] JIANG S, HU T, GILD J, et al. A new class of high-entropy perovskite oxides [J]. Scripta Materialia,2018,142:116-120. doi: 10.1016/j.scriptamat.2017.08.040 [24] 孟晓娟, 李丹丹, 贾翠超, 等. CH3NH3PbI3-xBrx薄膜的合成及光电性能 [J]. 燕山大学学报,2019,43(4):331-336.MENG Xiaojuan, LI Dandan, JIA Cuichao, et al. Synthesis and photoelectronic properties of CH3NH3PbI3-xBrx films [J]. Journal of Yanshan University,2019,43(4):331-336. [25] DABROWA J, STYGAR M, MLKULA, et al. Synthesis and microstructure of the (Co, Cr, Fe, Mn, Ni)3O4 high entropy oxide characterized by spinel structure [J]. Materials Letters,2018,216:32-36. doi: 10.1016/j.matlet.2017.12.148 [26] 顾俊峰, 邹冀, 张帆, 等. 高熵陶瓷材料研究进展 [J]. 中国材料进展,2019(9):855-865.GU Junfeng, ZOU Ji, ZHANG Fan, et al. Recent progress in high-entropy ceramic materials [J]. Materials China,2019(9):855-865. [27] DUPUY A, WANG X, SCHOENUNG J. Entropic phase transformation in nanocrystalline high entropy oxides [J]. Materials Research Letters,2019,7(2):60-67. doi: 10.1080/21663831.2018.1554605 [28] SARKAR A, DJENADIC R, USHARANI N, et al. Nanocrystalline multicomponent entropy stabilised transition metal oxides [J]. Journal of the European Ceramic Society,2017,37(2):747-754. doi: 10.1016/j.jeurceramsoc.2016.09.018 [29] MAO A, XIANG H, ZHANG Z, et al. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder [J]. Journal of Magnetism and Magnetic Materials,2019,484:245-252. doi: 10.1016/j.jmmm.2019.04.023 [30] BIESUZ M, SPIRIDIGLIOZZI L, DELL'AGLI G, et al. Synthesis and sintering of (Mg, Co, Ni, Cu, Zn)O entropy-stabilized oxides obtained by wet chemical methods [J]. Journal of Materials Science,2018,53(11):8074-8085. doi: 10.1007/s10853-018-2168-9 [31] HONG W, CHEN F, SHEN Q, et al. Microstructural evolution and mechanical properties of (Mg, Co, Ni, Cu, Zn)O high-entropy ceramics [J]. Journal of the American Ceramic Society,2019,102(4):2228-2237. [32] 李工, 崔鹏, 张丽军, 等. 高熵合金研究现状 [J]. 燕山大学学报,2018,42(2):95-104.LI Gong, CUI Peng, ZHANG Lijun, et al. Current studies of high entropy alloys [J]. Journal of Yanshan University,2018,42(2):95-104. [33] BRAUN J, ROST C, LIM M, et al. Charge-induced disorder controls the thermal conductivity of entropy-stabilized oxides [J]. Advanced Materials,2018,30(51):1805004. doi: 10.1002/adma.201805004 [34] WITTE R, SARKAR A, KRUK R, et al. High-entropy oxides: An emerging prospect for magnetic rare-earth transition metal perovskites [J]. Physical Review Materials,2019,3(3):034406. doi: 10.1103/PhysRevMaterials.3.034406 [35] MAO A, QUAN F, XIANG H, et al. Facile synthesis and ferrimagnetic property of spinel (CoCrFeMnNi)3O4 high-entropy oxide nanocrystalline powder [J]. Journal of Molecular Structure,2019,1194:11-18. doi: 10.1016/j.molstruc.2019.05.073 [36] DONG Y, REN K, LU Y, et al. High-entropy environmental barrier coating for the ceramic matrix composites [J]. Journal of the European Ceramic Society,2019,39(7):2574-2579. doi: 10.1016/j.jeurceramsoc.2019.02.022 [37] 邹芹, 关勇, 李艳国, 等. TiAl合金及其复合材料的研究进展与发展趋势 [J]. 燕山大学学报,2020,44(2):95-107.ZOU Qin, GUAN Yong, LI Yanguo, et al. Research progress and development trend of TiAl alloy and its composite materials [J]. Journal of Yanshan University,2020,44(2):95-107. [38] MAO A, XIANG H, ZHANG Z, et al. A new class of spinel high-entropy oxides with controllable magnetic properties [J]. Journal of Magnetism and Magnetic Materials,2020,497:165884. doi: 10.1016/j.jmmm.2019.165884 [39] 陈见, 尹周澜, 张衡中. Li、Mn掺杂对MgCoNiCuZnO5导电性能的影响 [J]. 有色金属工程,2019,9(8):1-6.CHEN Jian, YIN Zhoulan, ZHANG Hengzhong. Effect of Li and Mn doping on the conductivity of MgCoNiCuZnO5 [J]. Nonferrous Metals Engineering,2019,9(8):1-6. [40] 陈克丕, 李泽民, 马金旭, 等. 高熵陶瓷材料研究进展与展望 [J]. 陶瓷学报,2020(2):157-163.CHEN Kepi, LI Zemin, MA Jinxu, et al. Research progress and prospect of high-entropy ceramic materials [J]. Journal of Ceramics,2020(2):157-163. [41] CHEN H, FU J, ZHANG P, et al. Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability [J]. Journal of Materials Chemistry A,2018,6(24):11129-11133. doi: 10.1039/C8TA01772G [42] CHEN H, LIN W, ZHANG Z, et al. Mechanochemical synthesis of high entropy oxide materials under ambient conditions: Dispersion of catalysts via entropy maximization [J]. ACS Materials Letters,2019,1(1):83-88. doi: 10.1021/acsmaterialslett.9b00064 [43] EDALATI P, WANG Q, RAZAVI-KHOSROSHAHI H, et al. Photocatalytic hydrogen evolution on a high-entropy oxide [J]. Journal of Materials Chemistry A,2020,8(7):3814-3821. doi: 10.1039/C9TA12846H [44] ZHENG Y, YI Y, FAN M, et al. A high-entropy metal oxide as chemical anchor of polysulfide for lithium-sulfur batteries [J]. Energy Storage Materials,2019,23:678-683. doi: 10.1016/j.ensm.2019.02.030 [45] SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage [J]. Nature Communications,2018,9(1):3400. doi: 10.1038/s41467-018-05774-5 [46] WANG J, CUI Y, WANG Q, et al. Layered high-entropy oxide structures for reversible energy storage [J]. Energy,2020,1:1-7. -




 下载:
下载:


 邮件订阅
邮件订阅 RSS
RSS
